Photo/VCG
On August 1, local time, City of Hope, a top cancer treatment and research institution in the United States, announced that it has developed a molecular drug called AOH1996, which is in preclinical studies. This drug “seems to eliminate all tumors” while not harming healthy cells.
This news immediately attracted widespread attention. According to the latest paper published by the research team in Cell Chemical Biology, this targeted chemotherapy drug showed “great potential” in preclinical studies.
However, a Chinese representative of a large non-profit hospital in the United States told NBD: The anti-cancer drug announced by City of Hope is not a big news in the United States. Moreover, the drug is still far from actual application.
NBD noticed that since the drug is currently only in phase I clinical trials, it is at least several years away from being put into use. In addition, some professionals questioned that the support for AOH1996’s claim of “killing cancer cells” in the paper seems limited.
AOH1996 “selectively” kills cancer cells without damaging normal cells
The paper shows that City of Hope researchers tested AOH1996 in more than 70 cancer cell lines and concentrated normal control cells, and found that the drug selectively kills cancer cells by disrupting the normal reproductive cell cycle. AOH1996 is effective against laboratory-cultured cells from several different types of cancer (including breast cancer, prostate cancer, brain cancer, ovarian cancer and lung cancer). The drug prevents DNA-damaged cells from dividing and replicating defective DNA, thus causing cancer cell death, but does not interrupt the reproductive cycle of healthy cells.
It is reported that AOH1996 is named after Anna Olivia Healey, a cancer patient born in Indiana in 1996. Anna died of neuroblastoma at the age of 9, which is a very rare childhood cancer.
AOH1996 represents the highlight of City of Hope’s 20 years of research. The function of this new drug is to target a protein called PCNA, which is proliferating cell nuclear antigen. In its mutated form, PCNA helps cancerous tumors grow.
City of Hope official press release
Currently, AOH1996 drug is undergoing phase I clinical trials at City of Hope. Because AOH1996 killed cancer cells in several cancer cell lines, it brought hope that this drug could one day be used to treat breast cancer, prostate cancer, brain cancer, ovarian cancer, cervical cancer, skin cancer and lung cancer. This also means that this new drug may one day become a useful tool for combination therapy and developing new chemotherapy drugs.
The development of AOH1996 began with a daunting challenge: to create a molecule that can interact with the L126-Y133 region of PCNA, a protein that is essential for DNA replication and repair in enlarged tumors. The scientists turned to using advanced software to simulate different shapes and positions of molecules to determine the ideal structure that can bind to this region of PCNA.
This process is called computer modeling, and has become the cornerstone of modern drug discovery. It allows scientists to simulate and analyze millions of different interactions between molecules and specific protein targets. Through this method, they can predict the possible effects of each molecule and identify promising candidates for further study.
After 20 years of continuous simulation and analysis, AOH1996 appeared before the world today. This molecular drug showed the potential to inhibit PCNA activity, thereby disrupting DNA replication in cancer cells and inducing cell death. This discovery marked a major breakthrough in the project, because PCNA was previously considered an “undruggable” target due to its complex structure and important role in cell proliferation.
It’s just a start, it takes years to verify the benefits of new drugs
The researchers plan to further study the mechanism of action of AOH1996 to better understand this drug and improve the ongoing clinical trials. They wrote in the paper: “This study reports the anti-cancer activity of AOH1996 in multiple cancer cell lines and concentrated animal tumor models. But we acknowledge that positive animal study results do not always translate into success in treating cancer patients. Future clinical studies need to further determine (the drug’s) effectiveness for cancer treatment.”
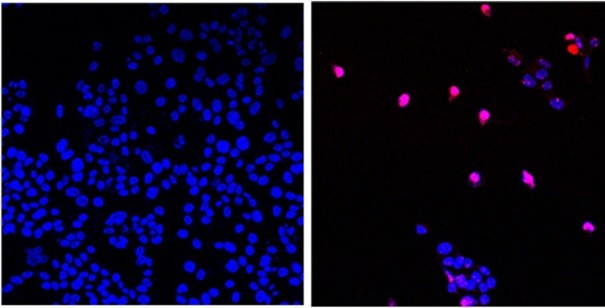
Untreated cancer cells (left) and cancer cells treated with AOH1996 (right) are undergoing programmed cell death (purple)
Image source: City of Hope
Foreign media reports said that even so, City of Hope’s discovery of AOH1996 is just a start. This molecular drug needs to be synthesized in the laboratory, tested on cancer cells in vitro, and evaluated for potential toxicity to normal cells. This rigorous testing process is essential for ensuring the safety and effectiveness of potential drugs before considering clinical trials.
City of Hope’s rigorous description of AOH1996 also indicates that they acknowledge that although early trials suggest that this therapy may be a breakthrough in the medical field, it is still in its early stages and cannot guarantee its success in human cancer patients. In addition, the cancer cells tested by the researchers were extracted from tumors removed from the laboratory, not from humans with cancer.
Although there is no reason to doubt that the efficacy of AOH1996 will not be replicated in clinical trials (involving human patients). But experts point out that about 90% of new anti-cancer drugs end in failure, including many that were very promising at first. The reason for the high failure rate of up to 90% is either because these drugs cannot adequately treat the target cancer cells compared with existing drugs, or because they have too many side effects.
Therefore, before AOH1996 is widely used, it still needs to undergo rigorous safety and effectiveness tests, as well as large-scale clinical trials.
Lawrence Young, a professor of molecular oncology at the University of Warwick in the UK, did not participate in this study, but he said he was skeptical of so-called cancer treatment “breakthroughs”. He pointed out that “this new drug looks very interesting, but it will take years to accurately test the possible benefits of this drug compared to conventional therapies. To prove the real benefits of a new drug, large-scale randomized trials are needed in thousands of patients, which is the so-called phase III clinical trial, which usually takes about 10 years.
Professor Dorothy Bennett of cell biology at St George’s College, University of London, thinks that the claim made by this team of scientists is not well supported. Dorothy Bennett pointed out that “the support for AOH1996’s claim of ‘killing cancer cells" in the paper seems limited. The paper only provides cell-killing data for 3 human cancer lines, and shows that some cancer cells in each culture were killed, not all. Similarly, tests on human tumors implanted in mice did not show that AOH1996 could kill cancer cells, but the growth rate of 3 tumors slowed down.”


 川公网安备 51019002001991号
川公网安备 51019002001991号





