According to WHO data, approximately 332 million people worldwide suffer from depression, with around one-third of patients showing poor response to conventional medications—classified as "treatment-resistant depression." A mental health survey in China indicates that the number of depression patients in the country stands at roughly 95 million.
However, depression treatment has long faced challenges such as clinicians understanding "what works but not why" and significant side effects for patients.
Currently, this puzzle plaguing the neuroscience community has been solved by a Chinese team.
On November 5th, the research findings from the laboratory of Luo Minmin, Director of the Chinese Institute for Brain Research, Beijing (hereinafter referred to as "CIBR"), were published on the website of the international academic journal Nature.

This study is the first in the world to confirm that the rapid antidepressant effects of both the drug ketamine and the physical intervention electroconvulsive therapy (ECT) are driven by a common mechanism—the adenosine signaling pathway in the medial prefrontal cortex (mPFC) of the brain.
This discovery provides a new biological target for depression treatment and spawns innovative therapeutic strategies. Building on this, Luo Minmin's team has designed candidate drugs with fewer side effects and simultaneously developed a non-pharmaceutical therapy—intermittent hypoxia (aIH), which has entered the clinical validation phase. Luo Minmin hopes it will become a widely accessible home therapeutic device.
Behind this achievement lies twelve years of exploration by Luo Minmin's team.
On November 9th, Luo Minmin accepted an exclusive interview with National Business Daily (hereinafter referred to as "NBD"), recounting the team's journey from early failures to an unexpected discovery in a 2019 experiment, and the current prospects for the parallel translation of pharmaceutical and non-pharmaceutical therapies.

Luo Minmin (Photo provided by the interviewee)
Chinese Team Finds "Master Switch" for Rapid Antidepressant Therapies
The core conclusion of the research from Luo Minmin's laboratory at CIBR points to a signaling molecule called adenosine.
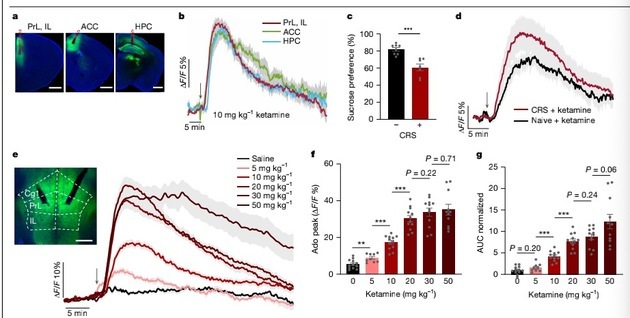
Using genetically encoded fluorescent probe technology, the team achieved real-time observation of adenosine signals in the brains of living animals for the first time. Results showed that both injection of low-dose ketamine and application of electroconvulsive stimulation triggered a rapid, substantial, and sustained increase in adenosine levels in the medial prefrontal cortex—a key brain region for emotion and advanced cognitive functions.
Explaining this phenomenon in the interview with NBD, Luo Minmin stated that ketamine and ECT reach the same goal through different pathways: the former inhibits cellular energy synthesis, while the latter increases neuronal energy consumption, both prompting the massive release of adenosine from cells "by different routes leading to the same destination."
To verify whether the adenosine signaling pathway plays a decisive role, the research team conducted "blocking" and "simulation" experiments. When adenosine signal reception in the brain was blocked through genetic or pharmacological means, the antidepressant effects of ketamine and ECT disappeared; conversely, direct activation of this signaling pathway mimicked antidepressant effects in animal models. This proves that the adenosine signaling pathway is the core hub driving the efficacy of these two therapies.
This discovery provides, for the first time, a unified biological mechanism for rapid antidepressant therapies, transitioning from past "empirical use" to an era of precision medicine with clear mechanisms, and offering a roadmap for the development of new drugs and therapies.
From "Unexpected Discovery" to Pathway Identification: Years of Exploration Finally Turned a Corner in 2019
"In 2013, I first heard in a presentation that ketamine has rapid antidepressant effects in humans," Luo Minmin recalled. His initial thought was: "Can we observe how ketamine affects the electrophysiological changes of nerve cells?"
However, the path was not smooth. A core challenge was that while ketamine's instantaneous impact on neural electrical activity was evident, how to link it to the antidepressant effects that only manifest hours later remained unclear.
"In the early years, several students in our laboratory used electrophysiological methods we were proficient in, but the experimental reproducibility was low," Luo Minmin admitted. "At that time, the field was filled with various theories—some said ketamine affects serotonin, others dopamine, opioid receptors, or the cannabinoid system—but we struggled to stably replicate these effects in our own laboratory."
By 2017, after four years of research, progress was minimal. Meanwhile, an industry-wide setback emerged: several major international pharmaceutical companies developing new antidepressants based on ketamine's traditional target (N-methyl-D-aspartate receptor, NMDAR) suffered successive failures in clinical trials. "That's when I started to doubt that the traditional approaches in this field might be flawed," Luo Minmin said. "We still had to persist and look for something new."
A turning point came from reading literature.
Clinical reports indicated that ketamine has significant anti-inflammatory effects, a discovery made far earlier than its application in antidepressant treatment. Luo Minmin seized on this, deciding to explore breakthroughs from this angle, which was generally overlooked by neurobiologists.
He told NBD: "I began to examine how ketamine affects effects that neurobiologists don't typically focus on, especially its anti-inflammatory properties."
In the laboratory, they found that ketamine's anti-inflammatory effects were highly reproducible—a stark contrast to the instability of electrophysiological experiments. Following this clue, a paper on ketamine's impact on adenosine levels in blood cells came to his attention. "I thought this was very interesting," Luo Minmin recalled. "There was already considerable literature showing that adenosine has antidepressant effects, including the antidepressant benefits of sleep deprivation and ketogenic diets, which are both related to adenosine."
The direction became clear, but new challenges soon arose.
Adenosine is a rapidly metabolized molecule in the brain, degraded within ten seconds, making it impossible for traditional biochemical detection methods to capture its dynamic changes. Luo Minmin's team tried various methods mentioned in the literature but failed. The research hit another snag.
The turning point occurred in May 2019.
At that time, Luo Minmin's laboratory collaborated with Professor Li Yulong's team from Peking University, who had developed genetically encoded fluorescent probes targeting adenosine. Le Chenyu, a postdoctoral fellow in Luo Minmin's team, conducted an experiment in mouse brains using the newly developed adenosine fluorescent probe.
"I clearly remember that day—May 20th, 2019," Le Chenyu said in a media interview. "I injected ketamine into the mouse, and within one minute, the fluorescent curve representing adenosine signals on the screen surged dramatically!"
"When I saw the data, I was shocked!" Luo Minmin described his initial reaction to the results. He immediately asked Le Chenyu to repeat the experiment. Two days later, stable replicated results made him realize this might not be a coincidence.
However, he remained cautious and arranged for another student to conduct independent verification. When the same clear fluorescent curve appeared again, Luo Minmin confirmed: "This could be something worth in-depth exploration." From 2013 to 2019, after more than six years of groping, the team finally found the door to the new mechanism.
Prospects for New Drug Development: Designing "Upgraded" Ketamine with Fewer Side Effects
"What's the biggest problem in our field? People are confined to publishing papers without telling a story that can infer new discoveries," Luo Minmin emphasized. The value of basic research ultimately lies in its ability to provide insights for clinical translation.
Finding the adenosine pathway—the "master switch"—is only the first step; more importantly, using this "roadmap" to create new therapies that can help patients. Designing new molecules that efficiently activate the adenosine pathway while having fewer side effects has become a feasible strategy.
To this end, Luo Minmin's team collaborated with Wang Xiaohui's team from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, which has extensive experience in small molecule synthesis. Taking the ketamine molecule as a blueprint, they modified multiple key sites, designing and synthesizing more than 30 new ketamine derivatives in a short period. These candidate molecules were then sent back to CIBR for screening based on "adenosine activation ability." Using fiber photometry, researchers could directly monitor the ability of each derivative to induce adenosine release in real-time in the brains of living mice.
In this round of screening, new molecules such as "dechloroketamine" (DCK) and its analogs performed exceptionally well. Animal experiment results showed that at a low dose of 2mg/kg, the adenosine release induced by DCK was comparable to or even exceeded that of high-dose ketamine (10mg/kg). In hyperactivity tests evaluating side effects, this dose of DCK only caused a slight increase in activity, lower than the significant hyperactivity induced by high-dose ketamine.
Luo Minmin explained to NBD: "With equivalent antidepressant effects, its side effects seem significantly reduced. But it's not a perfect drug—side effects still occur at higher doses, so there is room for improvement in the molecule itself. Currently, the priority is to reduce side effects."
Despite the promising prospects, Luo Minmin maintains the prudence of a scientist. "The development of small molecule drugs will definitely take a long time," he admitted in the interview. "We have applied for patents for the newly discovered molecules, but completing all preclinical studies and Phase I, II, and III clinical trials before finally launching them on the market will likely take 3 to 5 years."
Non-pharmaceutical Therapy Takes the Lead: Hopes for "Hypoxia Therapy" Device to Become a Home Treatment Tool
Compared with new drug development, which has a long cycle and high investment, there is another non-pharmaceutical therapy pathway based on the adenosine mechanism. The inspiration for this new pathway came from the analysis of the mechanism of ECT.
"Peking University Sixth Hospital is next to my office. I have also visited the hospital to watch them perform ECT. All the doctors said, 'Professor Luo, you should come see this—we do ECT every day and really want to understand the principle,'" Luo Minmin mentioned in the interview. Feedback from clinicians prompted him to expand his research to ECT.
Experiments confirmed that ECT also induces massive adenosine release by causing excessive neuronal excitement and energy consumption. However, ECT's strong irritation and potential memory damage are barriers to its application. "Can we find an alternative that doesn't use electroconvulsive therapy, as its side effects are too severe?" Luo Minmin pondered.
The team turned their attention to a physiological intervention method—intermittent hypoxia (aIH).
"If you don't get enough oxygen, adenosine is also produced; you don't necessarily need to take drugs," Luo Minmin revealed to NBD. The intermittent hypoxia therapy experiments were conducted from November to December 2024. The theory is that by having users breathe hypoxic air briefly and controllably, the brain can be safely induced to produce adenosine, thereby achieving antidepressant effects.
After theoretical verification, the team developed a prototype device for this therapy. The core of the device is a system that regulates the oxygen concentration of inhaled air, equipped with a blood oxygen saturation monitor to ensure safety.
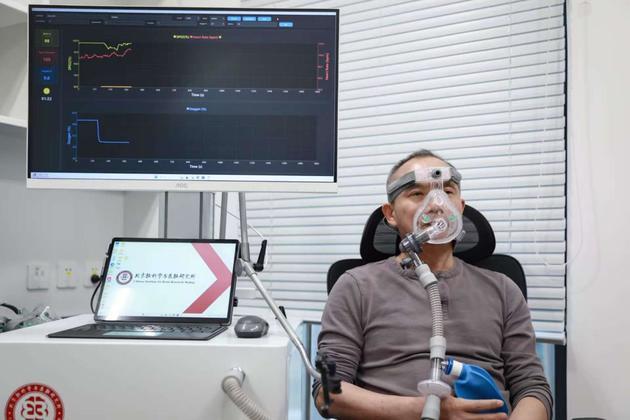
Luo Minmin testing the intermittent hypoxia therapy (Photo provided by the interviewee)
"Our (hypoxia therapy) is intermittent—several minutes of hypoxia followed by several minutes of normal oxygen—which further improves safety. The concentration is also dynamically adjusted and will not be too low," Luo Minmin said in the interview. Currently, this therapy has entered the clinical validation phase.
After the 2025 Spring Festival, Luo Minmin's team began collaborating with Beijing Anding Hospital, Capital Medical University, to conduct a double-blind controlled clinical trial on more than 30 patients with treatment-resistant depression to evaluate its safety and efficacy.
Luo Minmin stated that the team is currently awaiting the results of the clinical trial. Regarding the future of this non-pharmaceutical therapy, he expressed anticipation to NBD. "This device is not expensive, and the technology is not complex—it's about the size of an air purifier," Luo Minmin added. Converting this technology into a mass-produced product will not be difficult.
If the clinical trial ultimately confirms its effectiveness, it will mean that depression patients may, in the future, receive treatment at home through a non-invasive, convenient, and low-cost method.


 川公网安备 51019002001991号
川公网安备 51019002001991号





