According to CCTV News and Kyodo News reports on March 27, during a hearing held by Japan’s Ministry of Health, Labour and Welfare on March 26, Kobayashi Pharmaceutical Co. reported that another death might be linked to consumoption of red yeast rice dietary supplements.
The report stated that, there have now been two fatalities associated with the company’s red yeast rice products. The causal relationship between the deaths and the health products is still under investigation.
Additionally, as reported by Jiji Press, the Ministry of Health, Labour and Welfare has notified the relevant departments in Osaka City, where Kobayashi Pharmaceutical’s headquarters are located, to take measures such as disposal of the products in question, based on the Food Sanitation Act.

Photo/CCTV
Kobayashi’s Health Products in Question: Red Yeast Rice Hearing Conducted
On March 26, local time, the Ministry of Health, Labour and Welfare conducted a hearing regarding the red yeast rice ingredient used by Kobayashi Pharmaceutical, which may have compromised consumer health.
The hearing began around 16:00 local time and lasted for approximately three hours. The person in charge at Kobayashi Pharmaceutical presented detailed information about the issue. Based on the hearing and other information, the Ministry will decide whether Kobayashi Pharmaceutical has violated the Food Sanitation Act.
33 Illnesses Reported: Kobayashi Pharmaceutical Recalls Health Products
CCTV News recently reported that Kobayashi Pharmaceutical, a well-known Japanese pharmaceutical company, announced an emergency recall of three products containing red yeast rice after some consumers experienced kidney and other health issues post-consumption. On March 25, the company stated that 33 people had developed kidney diseases and other health conditions after taking the red yeast rice products, with 26 of them being hospitalized.

Photo/CCTV screenshot
On March 22, Kobayashi Pharmaceutical announced on its official website that some consumers had developed kidney diseases after using their red yeast rice products, with some patients requiring hospitalization and even dialysis.
Kobayashi Pharmaceutical found that some of the red yeast rice ingredients might contain unknown substances not within the company’s control after conducting an analysis.
The three products involved in the incident, as announced on the company’s official website, are “紅麹コレステヘルプ”(红麹胆固醇颗粒)“ナットウキナーゼ さらさら粒GOLD”“ナイシヘルプ+コレステロール”.
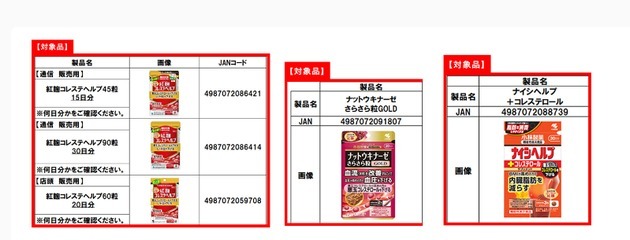
Kyodo News reported that the “Red Yeast Rice Cholesterol Granules” have been sold through e-commerce channels and stores since February 2021, with about 1.06 million bags sold by the end of February this year.
On March 25, Kobayashi Pharmaceutical announced that 33 people had developed kidney diseases and other health abnormalities after consuming their red yeast rice products, with 26 of them hospitalized.
Promoted for Cholesterol Control, Red Yeast Rice Products Removed from Official Stores
Kobayashi Pharmaceutical, a century-old Japanese pharmaceutical company, claims that red yeast rice dietary supplements are produced by fermenting steamed rice with red yeast mold. The company has promoted this ingredient as potentially lowering low-density lipoprotein cholesterol levels.
Kobayashi Pharmaceutical’s products are quite popular, with official online stores on several Chinese e-commerce platforms selling their products.
On March 22, Kobayashi Pharmaceutical issued a notice on its social media official, reminding consumers to stop consuming them.
As of now, the Red Yeast Rice Cholesterol Granules have been removed from the e-commerce platform mentioned in the notice, the official Kobayashi Pharmaceutical online store.

Following Kobayashi Pharmaceutical’s announcement of the emergency product recall, various Japanese food and beverage manufacturers have successively announced recalls of products containing red yeast rice produced by Kobayashi Pharmaceutical.
On March 24, Takara Shuzo Co., Ltd., a Japanese liquor manufacturer, announced that it would voluntarily recall about 96,000 bottles of a liquor product that used red yeast rice supplied by Kobayashi Pharmaceutical. There have been no reports of health damage related to Takara Shuzo’s product.
The liquor manufacturer stated that this particular liquor was only exported to Singapore and Taiwan, China and did not enter the mainland Chinese market.
According to Japanese media reports, about 20% of the red yeast rice produced by Kobayashi Pharmaceutical is used by the company itself, while the remaining approximately 80% is sold as raw materials to food production companies and others. The company’s red yeast rice ingredients have been sold to about 50 food and beverage manufacturers.
President of Kobayashi Pharmaceutical Apologized
On March 22, the president of Kobayashi Pharmaceutical attended a press conference and bowed.

Photo/CCTV
Japanese media reported that analysis results on March 16 had already indicated that some of the red yeast rice ingredients might contain unknown substances, but the company did not announce the recall until March 22. The president of Kobayashi Pharmaceutical admitted that the decision-making was slow.
Kobayashi Pharmaceutical saw 24 consecutive years of net profit growth
According to the Shenzhen Economic Daily, public records show that Kobayashi Pharmaceutical is a product development-oriented enterprise, mainly engaged in the research, development, production, and sales of pharmaceuticals, medical devices, daily necessities, and food. Initially founded as “Kobayashi Seidai-dō” in 1886, the company’s headquarters were located in Nagoya, Japan. Established in 1998, the company’s products are not only sold domestically in Japan but also exported to the United States, the United Kingdom, Southeast Asia, and other parts of the world. Shanghai Kobayashi Daily Chemical Co., Ltd. is a wholly-owned subsidiary of Kobayashi Pharmaceutical in China.
In China, Kobayashi Pharmaceutical’s main brands include the disposable hand warmer series “Nuanbaobao,” the fever-reducing cold patch series “Bingbaotie,” the fragrance series “Shuang Hua Lei” and “Xiang Ju Yuan,” among others.
According to the latest performance data disclosed on Kobayashi Pharmaceutical’s official Japanese website, the company’s sales for the first half of 2023 amounted to 73.6 billion yen (approximately 3.5 billion yuan), a 3.3% increase year-on-year; operating profit was 10.4 billion yen (approximately 500 million yuan), up 5.5% year-on-year; and net profit for the current period was 7.8 billion yen (approximately 370 million yuan), a 2.1% increase year-on-year.
It is noted that Kobayashi Pharmaceutical has achieved 24 consecutive years of net profit growth.
Cover image: CCTV


 川公网安备 51019002001991号
川公网安备 51019002001991号





